Link to all the blog posts on the paper
Weighing fullerenes
The first thing to do was to see if we could observe the higher fullerenes in fullerene soot otherwise we would not know what was happening to them. I got in contact with Dr Angus Grey from the Biomedical imaging centre at the University of Auckland. He had recently acquired a mass spectrometer for the biomedical imaging research unit to analyse proteins in biological samples such as brain slices. The mass spectrometer instrument the UltrafleXtreme is one of the highest resolution mass spectrometers in the world over a large mass range (the large mass range is needed to look at proteins or in our case fullerenes). Below is a short video showing the basic idea of using a laser to produce charged molecules (ions) which can be pushed with an electric field into the chamber. The heavier ions will be accelerated to a lower speed than the lighter ones so the lighter molecules will reach the detector before the heavier ions where they are detected (by the electrons they emit when they hit the detector). This timed flight is a very accurate method to determine the mass of heavy ions.
The instrument is fairly impressive taking up a room in the basement.
Below is a picture of the inside of the device. The large tube is where the molecules are accelerated into and then reflected back to the detector to increase the distance they travel.
 |
| Credit |
The schematic below shows the path of the charged molecules inside of the instrument. In the case of analysing fullerene molecules, the laser excites the fullerene cages and ejects an electron from the molecule this gives the fullerenes a positive charge which allows them to be manipulated inside the instrument using electric fields.
 |
| Credit: Bruker |
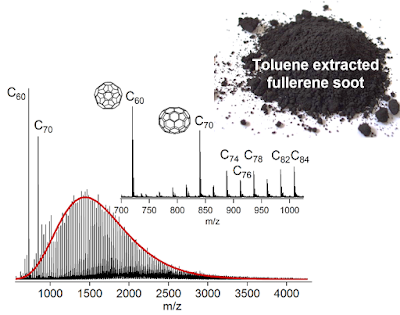
The mass spectrum which you measure on the computer looks like a collection of spikes they indicate the mass divided by the charge (m/z) of a molecule or collection of molecules with the same mass and charge (in our case the main ions from fullerenes have a single charge +1 so the spikes corresponds to the molecular mass of the fragments). The molecular mass of carbon is 12 g/mol so the spike/peak at 720 m/z is for C60 and the spike/peak at 840 m/z is C70. The higher fullerenes Cn>70 are much lower in concentration than C60 and C70 and are really hard to see so we added an inset showing the spectrum from 1000-5000 m/z multiplied by 150 in the figure below.
You can see a range of spikes between 1000-3000 m/z. They are separated by 24 m/z which corresponds to two carbon atoms. Fullerenes only lose or gain carbon in pairs due to the C2 dimers being very stable. So this comb of spikes separated by C2 indicates the comb are fullerenes. The laser is not continuously on by emits a short pulse of light and the power of that pulse can be reduced. We found we needed quite a high laser power to have enough energy in the laser pulse to see this comb of higher fullerenes. We think this is because there is quite a bit of C60 and C70 which absorbs most of the laser light. Unfortunately, the high laser power meant the C60 and C70 broke apart you can see this in the figure above if you look to the left of C60 and C70. C58 and C56, as well as C68 and C66 spikes, are seen indicating C60 and C70 are fragmenting and losing carbon. We also saw evidence for small fragments being formed. If you look at the figure below where we stacked mass spectra from lowest laser power (bottom) to highest laser power (top) see small fragments can be seen between 0-300 m/z being formed as you increase the laser power.
This is not what we want as this indicates the distribution is being changed from reactions happening after the laser has excited the molecules in the sample. Our focused changed to working out a way of reducing the amount of C60 and C70 in the fullerene soot to try and more easily observe the higher fullerenes. Due to C60 and C70's high symmetry, they are more easily dissolved in solvents like toluene while the higher fullerenes are quite insoluble. So we used a piece of glassware called a Soxhlet extractor to extract the C60 and C70 using the solvent toluene.
Some proper chemistry
Soxhlet extraction involves a really neat piece of glassware. You put your sample into a porous vial and place it into the middle section. The solvent, in our case toluene, is then added to a round bottom flask and heated up. The hot toluene vapour travels up a tube around the side of the middle section into a condenser which has cold water flowing around the outside of it. This cools down the toluene and forms liquid droplets which then drop onto the vial in the middle section. The fullerenes C60 and C70 dissolve into the toluene and give a nice pink colour. Once the middle section fills up and reaches the top of the siphon tube all of the toluene in the middle section siphons out, back into the round bottom flask. The neat thing is that each time the middle section fills with toluene it has fresh toluene without fullerenes present as the toluene vapor formed cannot hold any fullerenes due to the much higher vaporisation temperature of fullerenes. This improves the efficiency of the extraction. This extraction and siphoning repeats every 5-10 minutes and we left this running for 48 hours so this processes must have repeated over 250 times.
  |
| https://commons.wikimedia.org/wiki/File:Soxhlet_mechanism.gif |
By the end you can see the liquid in the round bottom flask has a nice pink colour full of C60 and C70. While the middle section is a clear liquid meaning all of the soluble C60 and C70 has been pulled out of the soot.
The distribution of higher fullerenes
We then analysed the soot again with the mass spectrometer (figure below) and you can see a much lower amounts of C60 and C70. This meant we could use much lower laser powers which gave no small fragments and no noticeable fragmentation. Below is a figure of what we found. A nice comb of spikes corresponding to fullerenes from C60 to C200.
The concentration of the higher fullerenes follows a function called a log-normal distribution (shown in red above) this indicates coalescence processes are involved in the formation mechanism. This log-normal size distribution is commonly associated with coalescence reactions where the smaller structure more easily coalescence due to weighing less or being more reactive.
| Credit: link |
In the next blog post, I will show what impact heating the fullerene soot with the C60 and C70 extracted has on the mass distribution of higher fullerenes.







No comments:
Post a Comment