tl;dr >1800 combustion scientists descended on Dublin last week to clean up combustion and pave the way for low carbon fuels. I gave my first talk at an international conference, where we suggested a new mechanism for soot formation based on curved aromatic molecules.
Over the last week, I have been in Dublin joining with a group of scientists from around the world to better understand combustion. This biannual conference has been going on since 1928 and has a strong community of scientists from universities and industry.
Why study combustion?
You might be fooled into thinking combustion is a dying field with the world transitioning from fossil fuels to electric transportation. However, this would be too small a view of the field of combustion. In the near future we need to be transitioning our current combustion engines to use low-carbon fuels such as biofuels, hydrogen and perhaps ammonia. This was a large part of the discussion at the conference. Carbon soot, or particulates from combustion, contribute substantially to global warming and by understanding the formation of soot many hope we will be able to quickly remove this contribution from combustion.
Combustion research doesn't just focus on combustion for transport and heating - surprisingly, many common materials are produced in flames. Car tires are full of very stable carbon particles which are formed in a furnace. These carbon blacks also hold onto the lithium in our batteries allowing us to store electricity in our laptop or cellphone. Practically all of the white titania (titanium dioxide) powder in paint is formed in a flame. The optical fibres that provide us with high-speed internet are made from very pure silica, which can only be produced in a flame. A large part of the symposium was focused on these materials and making new novel materials in flames.
What happens if we put the flame in there?
I saw talks from people who have put flames in space, super high pressure, vacuum chambers, synchrotrons, high magnetic fields, high voltage electric fields. In this section, I want to show some of the amazing experimental setups and instruments that were presented in talks and posters that are working on understanding soot formation.

 |
| Flame experiments in space |
 |
| Flame spray burners using biofuels imaged with lasers [Image Creidt: Kaust] |
 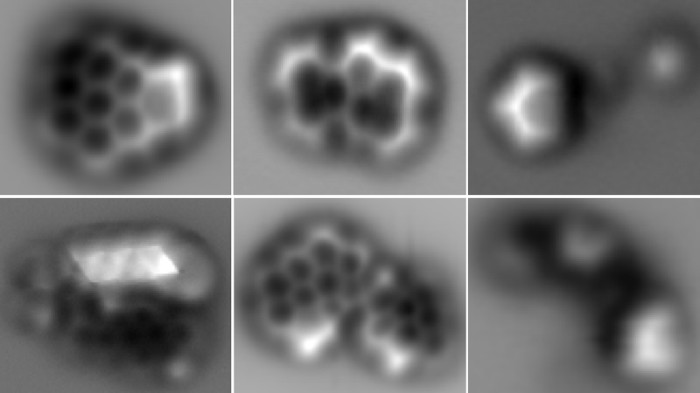 |
| Imaging of single soot molecules using atomic force microscopes. [Image credit:IBM Research-Zurich and F. Schulz et al./Proc. Combust. Inst.] |

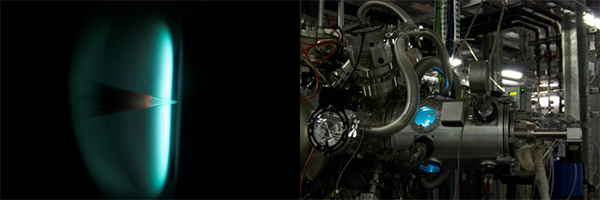 |
| Flame probed with an intense beam of x-rays from a stadium-sized instrument [Image Credit: Soilel] |
These new experimental setups are helping us to make leaps and bounds in our understanding of soot formation. Pentagonal rings and radicals were found to be prevalent in aromatic molecules in soot, which were previously thought to contain mainly hexagonal arrangements of carbons and could change the way these molecules self-assemble and react. However, we are still lacking some critical mechanistic understandings of soot formation.
Soot formation and curved aromatics
I presented a talk on the mechanism for soot formation and the impact of polar aromatic molecules. I have embedded a copy of the slides below.
We received some excellent questions and suggestions from the community on how to extend these preliminary suggestions in order to test this hypothesis.
An exciting presentation from Francesco Carbone showed evidence that positive ions and not negative ions continue to grow into the soot particles (some of the early work is found in a paper last year), which is what you would expect from the soot nucleation mechanism we are suggesting.
The great fun of this conference was meeting all of the other researchers from around the world and picking each other's brains over meals and pints of Guinness. Below is a picture of the Kraft research group which was the most numerous we have sent to a combustion symposium so far. Hopefully next symposium two years from now we will be closer to understanding how soot forms and whether these curved molecules contribute.




No comments:
Post a Comment