tl;dr In order to stop soot pollution, we need to know what reactions cause soot to form. We used the computer to work out how fast a variety of different reactions between soot molecules to see what reactions could be forming soot. Most of the reactions are too slow and suggest larger molecules are required.
Soot continues to be a problem for our climate by warming the atmosphere and melting ice. It also damages our bodies and causes significant health impacts. Some recent studies are coming out showing a strong relationship between polluted areas and places where the COVID-19 virus has taken many lives. For example in 66 administrative regions in Italy, Spain, France and Germany, 78% of COVID-19 deaths occurred in the five most polluted regions. (Ogen 2020). With a recent study based in the USA finding that for every 1 microgram per cubic metre of PM2.5 soot pollution is associated with an 11% increase in COVID-19 death rate? (Wu et al. Sci. Adv. 2020). Frustratingly we are still unable to describe how soot forms at the molecular scale and this is inhibiting our ability to reduce the emission of these toxic pollutants.
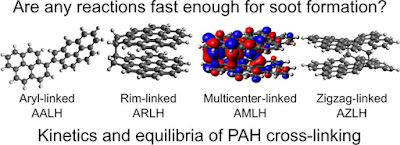
In this paper, my coworkers and I were able to run a series of calculations on the computer to systematically compare the speed of many reactions thought to happen in the flame. We refined a table of bond energies that we proposed in a previous paper (see this blog post) and by reordering the grid we found that we could categorise reactions into four main classes depending on the type of reactive site involved.




No comments:
Post a Comment