tl;dr Our new preprint "Carbonaceous nanoparticle formation in flames" is out.
The paper is now published in Progress in Energy and Combustion Science
A middle way can refer to many things. In common usage it
refers to a comprise between two positions. In philosophy or religion, it can
refer to a rejection of extremes as exemplified by Aristotle’s golden mean that
“every virue is a mean between two extremes, each of which is a vice”. In logic
it can refer to a fallacy - halfway between a lie and a truth is still a lie
and therefore some care is required in proposing such compromising positions.
In science it has been used for a variety of justifiable and unjustifiable
positions. One famous example being the middle way between physical scales and
another being a position we recently put forward for the formation of the
pollutant soot.
In the influential paper “The middle way” published in the Proceedings of the National Academy of Sciences of the USA in 2000, Laughlin et al. discussed the challenge in probing the scale between the atomic and macroscopic dimensions. In this mesoscopic region significant gaps exists in our understanding of how atoms and molecules interact, organise and form complex structures. This intermediate scale is too large to be measured by analytical chemical approaches and too small to be approached from the macroscale. Examples include protein folding, high temperature superconductors and disordered or topologically frustrated materials.
Our recent study on the formation of the pollutant soot illustrates the challenges probing the mesoscopic scale nicely. Figure 1 below shows a schematic of the transformation of fuel molecules into the pollutant soot. Only in the last 5 years have experimental techniques allowed for the aromatic soot precursor molecules as well as the earliest nanoparticles to both be directly imaged. Mass spectrometry has also allowed for the mass of the clustering molecules to be measured during soot formation. However, the mechanism by which these molecules cluster continues to baffle combustion scientists. The prize sought is the ability to understanding and potentially halt the emission of these toxic pollutants from internal combustion engines that damage almost every organ in our bodies as well as contribute to climate change.
 |
Figure 1 – Schematic for the transformation of fuel into
soot inside a flame with insets showing the experimental results from which the
schematic is derived. High resolution atomic force microscopy (HRAFM)from
Commodo et al. 2019, Helium ion microscopy (HIM) from Schnek et al. 2013, high
resolution transmission electron microscopy (HRTEM) from Martin et al. 2018 and
scanning electron microscopy (SEM) from Orion carbons.
Our modelling efforts also struggle to traverse the molecule
to nanoparticle transition in soot formation. There are two main classes of
models that have been proposed for soot formation. The first is physical
nucleation where aromatic molecules grow until the intermolecular interactions
between the molecules allows them to stick together and condense. The second is
chemical inception where bonds form between the molecular systems. Only
recently have accurate computational approaches been developed to explore these
suggestions.
Concerning physical nucleation, Prof. Kraft’s group worked
with the physical chemist Prof. Alston Misquitta (Queen Mary University) in the
2010s to accurately compute the intermolecular interactions between aromatic
species (using a symmetry adapted perturbation with a hybrid density functional
approach). From these results it was clear that the clustering species seen in
the flame are far too small to possess the significant intermolecular energies
required for physical nucleation mechanism. For my PhD, I explored electrical
enhancements to physical nucleation that arise from curved aromatic species
that possess a strong electric polarisation. While this electrical effect may
help explain the electrical control of soot formation it alone cannot justify a
nucleation mechanism either.
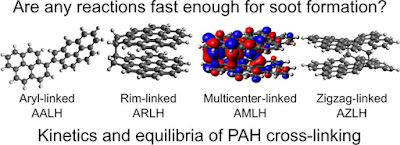
Concerning chemical inception, we recently undertook a
systematic study of the bonds that could form between reactive aromatic soot
precursors with Prof. Xiaoqing You’s group at Tsinghua University (made
possible by the CARES programme). This was only possible due to the direct
imaging of the reactive aromatics in 2019 (see Figure 1) and the recent
advances in density functional computational techniques optimised for radicals
(the meta hybrid GGA density functional method M06-2X). Figure 2 shows the
systematic comparison that was possible with such an approach for small
aromatic molecules. The green coloured grid squares correspond with thermally
stable species. Mr Angiras Menon was recently able to compute the rate at which
each of these crosslinks forms and compared them with the speed of soot
formation. We found that for these small species none of the crosslinks formed
sufficiently fast enough to explain the rapid clustering of molecules into soot
nanoparticles.
 |
| Figure 2 – Bond energy between various reactive aromatic soot precursors. Green indicates bonds that have enough thermal stability to be considered as important in flames. |
These detailed studies left us with the uncomfortable conclusion that the two main routes proposed for soot formation were unable to describe it. However, something did catch our attention crosslinks that allowed the molecules to both bond and stack, see Figure 2 B), C) and D) sites. This opened up another possibility that both physical and chemical mechanisms could cooperatively contribute to soot formation. Upon exploring these possibilities, we found that π-radicals on five membered rings, site B), formed highly localised states that did not become deactivated as the molecule grew in size, unlike their hexagonal ring equivalent, thereby remaining highly reactive. This allowed for an additive contribution between the physical interactions and the chemical bond only in these so-called aromatic rim-linked hydrocarbons (ARLH). Figure 3 shows the various mechanisms placed on a C/H versus molecular weight schematic to show the middle way suggested.
 |
Figure 3 – A middle way is schematically shown between physical and chemical mechanisms for soot formation. |
As mentioned at the beginning of this article claims to
middle ways are poor arguments unless they can be justified. Currently, we have
shown that the addition of physical interactions and chemical bonding considerably
increases the thermodynamic stability of aromatic rim-linked hydrocarbons.
However, we have yet to show that such species can explain the rapid formation
of soot in the flame. This requires the collision efficiency between these
species and the concentration of the localised π-radicals on five-membered rings to
be determined. Experiments are underway in the community to probe such species
and close this missing gap between the micro and mesoscale of soot formation.