tl:dr
We still don't know how soot forms and this is stopping us from eliminating it from internal combustion engines and furnaces. Recently, the molecules present, just before soot formation, were directly imaged. For the first time, many of the reactive edges could be seen. In this work, we computationally screened these reactive edges. We then considered all possible crosslinks between these edges. We discovered a new crosslink that allows the molecules to be stabilised by physically stacking on top of each other and then becoming bonded at their rim. This could help explain the rapid growth of soot particles in the flames and lead to new ways to clean up combusiton.
We have just published a new paper in the Journal of Physical Chemistry C. Here is the infographic/abstract figure.
Reactive molecules involved in soot formation
At the 37th International Symposium on Combustion, an extraordinary paper was presented directly imaging the molecules present just prior to soot formation. In the case of most of these aromatic molecules it is the edge that is the most reactive and over the years many suggestions have been made but never directly observed. So here they are.
Here are some of the most exciting findings.
Firstly, some were found to be crosslinked suggesting reactions between radicals and molecules during soot formation. This contradicted a commonly held view that only physical interactions and not chemical reactions were involved.
Secondly, there were lots and lots of pentagonal rings. Out of the 49 molecules (above 4 rings) imaged 28 contained at least one pentagonal ring and 12 contained two pentagonal rings on their rim. Previously only six-membered rings were thought to be stable at flame temperature.
Thirdly, species very close to curvature integration were found. While curved 3D were unable to be imaged using this technique at present, the presence of the almost curved molecules was encouraging for our suggestion of curved aromatic molecules being important in soot formation as
I have previously discussed in this blog.
Finally, some of these pentagonal rings were found to have hydrogen added to them. This forms a completely new radical type (–CH=CH– + H → –C•HCH2– which we found formed a localised π-radical).
Given the wide range of interesting new molecules that were found we considered how their reactivities compared.
We made use of computational chemistry to compute the energy needed to remove an electron from a particular spot on the "surface" of the molecular surface (average local ionisation energy). This told us how likely it was to form a bond with another molecule and therefore allowed us to compare their reactivities.
One significant surprise was the reactivity of pentagonal rings and a new localised π-radical on pentagonal rings
B).
Many reactions are important in the flame
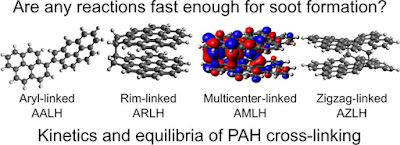
Now that the reactive sites were characterised we considered which crosslinks between them could be important in the flame. Below is a figure of the crosslink energies. The green indicates bonds that are strong enough to persist at the high temperatures within a flame.
Most crosslinks are well-known mechanisms, however, the reactions with the localised π-radicals B) were completely novel.
A new type of bonding is possible - rim-bonding
Most of the ideas for how the molecules in flames come together to form soot particles have been
either stacked physically interacting interactions
or chemical bonds in a long polymer that did not stack. However, the localised π-radicals
B) allows for stacked and bonded structures that are strongly bound.
This could allow molecules to rapidly condense and then crosslink which could explain the rapid growth of soot. Below is a drawing of how such a cluster could form we are calling an aromatic rim-linked hydrocarbon.
We need to figure out the concentration of this reactive site in the flame. We also need to compare how all of the possible crosslinks contribute to soot formation. Once this is achieved we can consider how to stop particular reactive sites from being made and reduce soot emissions.