I recently gave a presentation at a Pint of Science event in Singapore entitled "Combustion Science for Climate Solutions". Here are the slides with my transcript added into the slides.
Showing posts with label carbon capture. Show all posts
Showing posts with label carbon capture. Show all posts
Wednesday, 29 May 2019
Wednesday, 13 March 2019
How are the atoms arranged in charcoal?
In brief
- Charcoal is the black carbon product produced from heating biomass in a low oxygen environment.
- Why would we be interested in studying charcoal? It has recently been suggested as a potential carbon dioxide storage method to combat climate change (called biochar in this capacity). Instead of the photosynthetically trapped carbon dioxide being released when waste biomass decomposes it is trapped by carbonisation into stable biochar that will not break down for thousands of years. One advantage is that it can be sold as it can improve soil fertility. We need to understand the nanostructure of charcoal in order to understand how long it is stable in the ground and how best to optimise its properties. Charcoal can also be used in electronic applications and
- The currently understood nanostructure of charcoal is that it is made up sheets of carbon atoms in a "chicken wire" or hexagonal arrangement. These sheet-like molecules then stack into small graphitic disordered crystals. Below is a picture of some of these stacked regions in a char made from resin.
  |
| (Top) Model of stacked ribbons of carbon (Bottom) Ribbon-like graphene structures imaged in char [Guo et al. 2012]. Used with permission from Wiley. |
- Some of the highest magnification electron microscopes have found evidence for different nanostructures not planar but curved sheet-like carbon sheets where the curvature arises from non-hexagonal rings that warp the sheets.

Non-hexagonal rings imaged in chars indicating curvature [Guo et al. 2012]. Credit permission granted from Wiley - When scientists see curved carbon nanostructures the first thing that comes to our minds is the most famous curved carbon structures - fullerenes which are cages of carbon that form a spherical net. The most well known curved carbon molecule is C60 buckminsterfullerene with atoms arranged in a similar manner to the intersection of seams in a soccer ball with 20 hexagonal rings, and 12 pentagonal rings of carbon. Given the presence of non-hexagonal rings, many suggested the nanostructure should be fullerene-like.
 |
| C60 Buckminsterfullerene Credit |
- If charcoal is fullerene-like many researchers expected to see C60 as it was thought to be a stable form of carbon as it is readily produced in high-temperature carbon arcs, but none could be found.
- We produced some high-quality charcoal in a gasifier, see my other blog post on gasification for more information. But for this study, it served to produce high-quality charcoal with a well-defined nanostructure so no tar or soot stuck to the surface.
Gasifier was based on the Microlab gasifier from Fluidyne Gasification Ltd. - We used some of the most precise machines in the world to weigh the molecules in charcoal the Fourier Transform Ion Cyclotron Resonance Mass spectrometer (here is a video if you are curious about how it works from one of the authors Prof. Marshall). We did not find any C60 or C70 in gasification charcoal as has been found before. We did however found a common ion in many charcoals (mass to charge ratio of m/z 701) which we previously thought could be part of the nanostructure as it is near to that of C60 (m/z 720), but we found this to be an unstable breakdown product and not a molecule that lasted upon heating.

Ultra high resolution mass spectrometer - Using a different mass spectrometer that used a laser beam to ablate the sample and create charge molecules we could look at some heavier species and consider the nanostructure. We found a collection of molecules (peaks) that matched what we had found previously in a very curved carbon prepared from C60 arc-carbon that had been heated (see my previous post on these experiments).

Mass spectrum from charcoal showing oxygenated fragments
 |
| Mass spectrum from heated and oxygenated fullerene arc-carbon showing similar oxygenated species. |
- We found oxygen was present in all of these structures and a very similar set of molecules were found, which we could not reproduce repeating the experiment with graphite. This indicated that charcoal shares a curved oxygenated nanostructure with heat treated arc-carbon.
- A model was developed to explore the presence of non-hexagonal rings in a 3D graphene network.
 |
| Stacked fulleroid-like model of the surface of charcoal showing the integration of non-hexagonal rings |
- We are now working on understanding how this curvature is integrated into the structure and what the topology (shape) of these sheets are. We also want to apply this understanding to improve technologies that rely on these materials such as carbon capture using biochar, water purification with activated carbons and energy storage applications like electrodes in batteries and supercapacitors.
This project spanned a decade and involved the help of many others. I want to thank Mr Doug Williams (Fluidyne Gasification Limited) for his advice in designing and building the gasifier and Mr Peter Wilkinson (Wilkinson Transport Engineers) for allowing me access to the workshop to construct the gasifier. Prof. Brian Nicholson (University of Waikato) for allowing me access to the laboratory space and instruments. I would also like to thank Prof. Robert Curl (Rice University) for putting me in contact with the late Prof. Harry Kroto who arranged for the application of the FT-ICR MS experiments with the group at Florida State University. Finally, I would like to thank Assoc. Prof. Nigel Marks, Dr Irene Suarez-Martinez and Dr Carla de Toma ́s (Curtin University) for providing the annealed molecular dynamics models online, which were used and modified to construct the model seen above.
Monday, 5 December 2016
Gasification and carbon capture
In 2006, I became interested in gasification as a way of generating energy from biomass while storing atmospheric carbon in the ground. I thought I would explain some of the experiments I did and some of the interesting things I found out.
What is gasification?
Gasification is the process of turning biomass, such as wood, into a fuel gas that an internal combustion engine can run on. Complete combustion of biomass produces water and carbon dioxide, but by restricting the amount of air allowed into the reactor you can produce an incompletely combusted gas made up of carbon monoxide, methane and hydrogen. This can then be piped into a normal spark ignition engine and used similarly to LPG.
There are four steps in gasification:
- Drying - The fuel is heated and water is removed from the biomass;
- Pyrolysis - The fuel heated without any oxygen breaks down and forms small volatile compounds (called tar or bio-oil) and solid charcoal;
- Combustion - The tar and charcoal are burnt in a small amount of oxygen from the air, generating heat for the entire process;
- Reduction - The amount of oxygen quickly runs out and the water and carbon dioxide are reacted on the hot charcoal surface to produce carbon monoxide and hydrogen.
 |
| Photo Credit: GEK |
Some of the benefits of gasification, as opposed to combustion on an open fire, includes the increased fuel efficiency, as combustion is much more efficient and clean when using a gas instead of a solid fuel. The conversion of biomass to electricity using simple combustion requires steam turbines which are only economical on a large scale. The ability to power an engine that can drive a generator means it is also a low-cost method to generate small scale power. By adding the charcoal that is generated to the soil, the entire process can be carbon negative by trapping the CO2 the tree took in during its growth and locking it away in a stable form of carbon charcoal.
These types of gasifiers were heavily deployed (in over a million vehicles) in Europe during WWII when fossil fuels were in limited supply. My favourite photo from this time is a picture of a tank powered by a gasifier.
 |
| Photo credit |
I built two different types of gasifiers - a gasifier stove and a downdraft gasifier, both of which I will outline below.
Gasifier stove
The first gasifier I built was a gasifier stove. The geometry of the gasifier is called a top-lit updraft gasifier (TLUD). This means the fuel is combusted from the top with the air moving up through the fuel. The diagram below shows the working principles.
 |
| Photo credit |
The fuel is lit from the top and air is supplied from the bottom. A flame front (migrating pyrolytic front) moves down through the fuel. The tar and water are pulled through the hot bed of coals, helping to break down some of the tar. Secondary air is then injected into the top of the reaction chamber which allows the fuel gas to burn cleanly. The stove was built from a computer supply box and used a forced draft from a computer fan which I powered on 12V DC. I mainly ran the stove on wood chips but I also used it to test the heat content of different fuels by heating water placed on top of the stove.
The stoves are not just a curiosity; thousands of them are being built and used in developing countries to improve the air quality for those who rely on solid fuels for cooking. The video below explains.
I also made use of the stove to study the combustion of algae during a summer research project working with Dr Rupert Craggs from National Institute of Water and Atmospheric Research (NIWA) in New Zealand. The algae were grown in open raceway ponds which used waste water to feed the algae.
 |
| Photo Credit: NIWA |
I made use of a non-woven geotextile to dry the algae from the 98% water content down to 7-12 wt% which is suitable for combustion. The higher heating value for the algae was 23.06 MJ/kg compared with wood at 14-17 MJ/kg. The dried algae formed flakes which made for excellent fuel and allowed for combustion in the gasifier stove. One thing I didn't measure was the emissions, as the high nitrogen content would suggest a large amount of nitrous oxide could be generated.
We published the results in a conference proceedings in 2010. https://www.waternz.org.nz/Article?Action=View&Article_id=786. In particular, we looked at the potential for algae to be carbonised to biochar to be a stable carbon sink.
Discovery model gasifier
The discovery model gasifier is a downdraft gasifier. This means the air is injected in the bottom and drawn down. The design of the gasifier is based on the Pacific class gasifier from a New Zealand company called Fluidyne. I scaled it down so that it could power a 660 cc engine at 1500 rpm outputting 3 kW of energy. This required a gas output of 9024 m3/hr of wood gas with a wood consumption of 4.19kg/hr. I initially had a 1kg hopper which allowed for a short test run of around 20 minutes. The design of the gasifier is really quite interesting and was designed to be built at a very low cost (a diagram of the gasifier is shown below). The fuel is loaded into the top and moves down as it is consumed. The fuel is dried and is broken down to tar and charcoal in the pyrolysis zone. Air is then injected through three nozzles and allows for combustion. A tube then comes up through the charcoal into the oxidation zone. Many gasifier designs make use of a metal throat that mechanically constricts the fuel. However, this throat can melt as the high temperatures are hot enough to melt steel. This gasifier makes use of the charcoal itself to act as the throat and the insulation allowing for low-cost materials to be used. As the carbon dioxide and water enter the tube the hot charcoal, in the absence of air, produces carbon monoxide and hydrogen.
The biggest advantage of a downdraft gasifier design is that all of the tar must go through the oxidation zone and then the reduction zone. This makes the fuel gas generated from these types of gasifiers very clean.
Here is picture inside the reactor with the constriction tube and the nozzles (the bolts are being stored there and are not used during operation). You can also see the diesel glow plug I used to start the gasifier in the top right corner.
The fuel I used was mainly wood chips or small wood rounds from the garden. The fuel gas then passed through a series of cleaning stages to prepare it for the engine. I used a blast tube to remove the large particles and some of the soot. A cyclone particle separator was made to remove the micron-sized soot particles. Cooling tubes were used to condense the water out of the gas and to generally reduce the temperature of the gas as well as to increase the density of the fuel gas. Finally, it went through a sawdust filter to remove any particles or tar that were missed in the previous stages. I later replaced the sawdust filter with a bag filter which could be cleaned and reused.
The gasifier was designed for a large generator, which I didn't end up finishing, but I did some preliminary tests with a smaller generator. Here is an interview I did where I started up the gasifier and ran the engine.
One of the important aspects of making the gasifier work well (i.e. tar free) was to adjust the height of the reduction tube so that it was inside the oxidation zone and the grate height to allow the fuel to flow. Two good checks for a tar free operation was a blue flame (meaning no hydrocarbons in the fuel) and no hydrocarbons in the condensate from the fuel gas cooler. As you can see from the picture above I got close to correctly tuning the gasifier however Fluidyne's Andes class gasifier flaring shows a really excellently tuned gasifier with only carbon monoxide and hydrogen burning.
 |
| Fluidyne |
The microlab gasifier was built by Fluidyne in 2011 and is the same size as the discovery model gasifier but with two cyclones and is now being used for research at the University of Ulster.
 |
| Microlab gasifier |
I have Doug Williams from Fluidyne to thank for showing me how to build and operate gasifiers. I also have to thank Peter Wilkinson from Wilkinson Transport Engineers, who allowed me to use his workshop and materials to build the gasifier.
Continued interest
My PhD research is on combustion, global warming and reducing soot emissions from engines so this still interests me greatly. Gasification of biomass is one of the key technologies for controlling the amount of carbon dioxide in the atmosphere. This is often referred to as bioenergy, with carbon capture and storage (BECCS). CO2 is captured by trees and the CO2 released during burning can be stored, making the process carbon negative.
 |
| Photo Credit: Drax Power |
A second option is to burn some of the carbon to CO2 and to store the rest of the carbon as solid charcoal. This is called bioenergy-biochar systems (BEBCS). This does not sequester all of the carbon but as the charcoal is easier to handle and when added to the soil (referred to as biochar) can improve the holding of nutrients. This process is cheaper as the biochar can be sold to offset the cost.
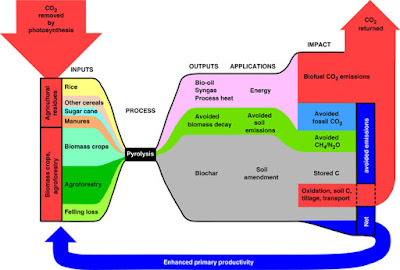 |
| Photo credit |
A recent paper on BEBCS compared with BECCS showed that in the short term, the former would be easier and cheaper to deploy. Biochar added to the soil also significantly reduces the amount of nitrous oxide emitted (as nitrous oxide comes from fertilisers and is ~300 times more potent a greenhouse gas than CO2).
I will probably be writing more about biochar in the future, but feel free to ask any questions about gasifiers.
Sunday, 17 April 2016
Sifting for carbon dioxide
Some colleagues and I have just had a paper accepted for publication and with a title like "Enhancement of Chain Rigidity and Gas Transport Performance for Polymers of Intrinsic Microporosity via Intramolecular Locking of the Spiro-carbon", I thought I would explain it in a more easy to understand format.
In short
- If we could filter out $CO_{2}$ from the emissions of powerplants we would be able to collect and economically pump it into underground storage without having to compress all of the other gases emitted from the powerplant, such as nitrogen (which can be up to 60%).
- New plastics have been recently made called 'polymers of instrinic microporosity' (PIM). They can let through $CO_{2}$ selectively, but more importantly they are very permeable which means they can separate out $CO_{2}$ on an industrial scale.
- Jianyong Jin (University of Auckland) and his team found a way to make the best PIM so far by introducing a locking mechanism between the molecules.
- To show how this locking mechanism improves the polymer, I used computer simulations to show that the lock increased the rigidity of the polymer and also produced the optimal geometry for the polymer. This made the pores just the right size for the $CO_{2}$ to pass through, and the large rigidity made it selective and very permeable.
- Here is a video of a similar material and the sort of separations that this polymer enables.
What is the most interesting detail if you are a scientist?
The most interesting detail for scientists is the idea of locking polymer molecules using an intermolecular locking mechanism. Many different polymer systems rely on the interplay between entropy and enthalpy. By locking the polymer backbone you can play with how the polymer packs and distributes its vibrational energy. One really interesting application we comment on in the paper is locking protein molecules using intramolecular locks. We are very interested in exploring this idea with other groups.
From a materials point of view, the idea of engineering the size of the pore by changing the geometry of the linkage is an interesting concept. You can go straight from a chemical structure to a material property.
Overview of the paper with some further details
The polymer monomer that was locked is called a spirobisindane, SBI for short, formed by adding bromine onto the 6-membered rings and then performing a silver oxidation which forms an ester bridge. This forms an 8-membered ring which bridges the weakly bonded spiro centre. Here is what it looks like with a 2D drawing.
 |
| Link |
It looks quite ridiculous in a two dimensional drawing. How can those two atoms be linked all the way across by an oxygen atom? Well, looking at the 3D drawing it makes a lot more sense, the 6-membered rings are actually a lot closer together than you might think and the bridge is only slightly strained. I have coloured the different rings so the 5-membered rings are coloured blue, the 6-membered rings are coloured pink and the 8-membered ring is coloured yellow (this also helps when comparing with the 2D representation shown earlier).
The image above is actually a 3D model you can rotate in the web page so click and hold on the picture and you can rotate you can also zoom in with the mouse wheel. Thanks to molview.org for the plugin.
The polymer, made up of thousands of repeats of the monomer, forms a very rigid structure which packs very poorly leaving lots of space (pores) for the gases to permeate through. With PIMs you want to optimise the pore size for the molecule you want to sort and also it is important this pore doesn't change size by much. Thermal energy from the polymer being at room temperature causes the pores to change size as the polymers move around. Changing the pore size reduces the selectivity for $CO_{2}$ over other gases so one method that has been used in the past is to rigidify the polymer. By replacing the spirobisindane centre with something more rigid you can make it more selective. Many of these attempts, however, changed the pore geometry so it was no longer big enough for $CO_{2}$. So what we have done was to rigidify a particular PIM, PIM-1, which we know has a good geometry for $CO_{2}$ separation, while keeping the large pore size.
The polymer, made up of thousands of repeats of the monomer, forms a very rigid structure which packs very poorly leaving lots of space (pores) for the gases to permeate through. With PIMs you want to optimise the pore size for the molecule you want to sort and also it is important this pore doesn't change size by much. Thermal energy from the polymer being at room temperature causes the pores to change size as the polymers move around. Changing the pore size reduces the selectivity for $CO_{2}$ over other gases so one method that has been used in the past is to rigidify the polymer. By replacing the spirobisindane centre with something more rigid you can make it more selective. Many of these attempts, however, changed the pore geometry so it was no longer big enough for $CO_{2}$. So what we have done was to rigidify a particular PIM, PIM-1, which we know has a good geometry for $CO_{2}$ separation, while keeping the large pore size.
To show how this intramolecular lock improves the rigidity of the polymer I simulated the movement of the polymer in the computer. This heats the polymer, causing it to vibrate and the rigidity is tracked by looking at the distance between the atoms at the end of the chain (end-to-end distance). This fluctuates over time performing a repetitive motion, almost like a snake. The polymer that is more rigid will have the less movement over time. There is a plot of the end-to-end distance over time (a) and also the bar graph showing the frequency of the end-to-end distances being at a certain distance.
 |
| Link |
What we found was that the unlocked polymer, labelled SBI, was much more mobile and the end-to-end distance varied a lot more than for the locked polymer.
Using a more advanced technique (taking into account the quantum mechanical description of all of the electrons in the molecule) we compared how much more rigid this locked PIM was, compared with other methods used to rigidify the polymer. The plots you see below show how the potential energy increases, from the baseline value, as I forcefully twist the polymer in the computer. Think of it like a spring - as it is twisted past its natural equilibrium, it will want to spring back. I actually used the equation for a spring to describe how rigid each polymer is. When describing a spring there is something called the spring constant $k_{ijkl}$ - the larger this value, the more the energy increases with the deflection from the equilibrium angle $\phi_0$. The equation for the spring's potential energy is a parabola:
$$E_{spring}=k_{ijkl}(\phi_{ijkl}-\phi_{0})^{2}$$
Fitting the spring model to the potential energy plots I calculated for the different polymers allowed us to compare with other linkages. We showed that there was a 230% increase in the rigidity for the locked SBI compared with the unlocked SBI. It also showed there are other linkages that are actually more rigid. However the locked SBI has both the correct geometry to get the right pores and has reasonably large rigidity.
Excellent gas separation experiments were performed by Tim Merkel and Sylvie Thomas at the Membrane Technology and Research, Inc. in the US. These are called Robeson plots and have selectivity on the y axis and permeability on the x axis. It was good to have a selective polymer but for industrial scale separations the real winner is having a permeable polymer. A permeable polymer allows for industrial scale separations.
 |
| Link |
All of the black open circles are common polymers. They have a trend that Robeson saw - as you increase their permeabilty you decrease their selectivity. He set an upper bound, the black line, that was not surpassed until these rigid polymers of instrinic microporosity were developed. You can see that for these two gas pairs $O_{2}/N_{2}$ and $CO_{2}/CH_{4}$ PIM-C1 is the most permeable polymer above the line. The first gas pair $O_{2}/N_{2}$ is useful for separating oxygen from the air which is mainly used to generate oxygen in medical applications. The second gas pair $CO_{2}/CH_{4}$ is important for removing carbon dioxide from natural gas which means you only need to transport the methane not the carbon dioxide as well; this is called natural gas sweetening. However, the important application is removing $CO_{2}$ from factory emissions. This is the $CO_{2}/N_{2}$ pair, which we didn't show in the paper, so I plotted it below.
I plotted the data with a linear scale and a log-log scale (which is most often used) as it might be more intuitive to see the data on a linear scale. You can see you get some good selectivity and a huge permeability for $CO_{2}$ which is exactly what we wanted for greenhouse gas capture. So the take home message is that careful design of the molecules leads to a huge change in the material properties and from a chemist's point of view, this was very exciting.
Subscribe to:
Posts (Atom)





















